Abstract
INTRODUCTION: Oral-AZA was approved in the United States in September 2020 for continued treatment (Tx) of adult patients (pts) with AML in first remission after intensive chemotherapy (IC). The phase 3 QUAZAR AML-001 trial showed that Oral-AZA significantly improved overall and relapse-free survival (OS/RFS) vs placebo (PBO) and was generally well-tolerated (Wei, NEJM 2020). In the phase 3 HOVON97 trial, subcutaneous (SC) AZA maintenance Tx significantly improved disease-free survival vs observation, but did not show an OS benefit (Huls, Blood 2019). Although Oral-AZA and SC AZA contain the same active drug, they are not bioequivalent. Oral administration allows extended exposure to lower drug concentrations, which could increase the number of diseased progenitor cells exposed to AZA and maximize therapeutic effects (eg, drug incorporation into RNA leading to apoptosis), and may improve tolerability by decreasing AZA-related exacerbations of existing cytopenias.
In the absence of head-to-head trials, a matching adjusted indirect comparison (MAIC), which adjusts for potential Tx effect modifiers, was previously conducted to evaluate the relative OS benefit with Oral-AZA vs SC AZA (Chen, EHA 2021). This MAIC was performed to determine the relative safety profile and health care resource utilization (HCRU) with Oral-AZA vs SC AZA maintenance therapy in pts with AML in first remission after IC.
METHODS: Anchored MAIC analyses of serious Tx-emergent adverse events (SAEs) and HCRU were performed using individual pt data (IPD) from QUAZAR AML-001 to match to baseline (BL) summary statistics from HOVON97. IPD from QUAZAR AML-001 were removed when a pt did not meet HOVON97 eligibility criteria. Remaining QUAZAR AML-001 pt data were weighted using a method-of-moments propensity score model. Matched and adjusted variables were BL age, platelet count, neutrophil count, ECOG performance score, cytogenetic risk, and response status. Analysis endpoints were times to first SAE, hospitalization, and platelet or red blood cell (RBC) transfusion. In HOVON97, pts could receive SC AZA for up to 12 cycles; therefore, QUAZAR AML-001 outcomes were limited to the first 12 cycles of Tx. Hospitalizations for SC AZA administration only in HOVON97 were excluded from analyses.
Endpoints are adjusted for pt-years of drug exposure and compared between studies by calculating relative risks (RR). Exposure to SC AZA in HOVON97 was imputed based on the number of pts entering each Tx cycle, and relies on the assumption that events/censoring happened at the end of the cycle and led to Tx discontinuation. Sensitivity analyses of model robustness relaxed these assumptions, comparing only the difference in cumulative hazard vs PBO/observation using a cloglog link binomial model.
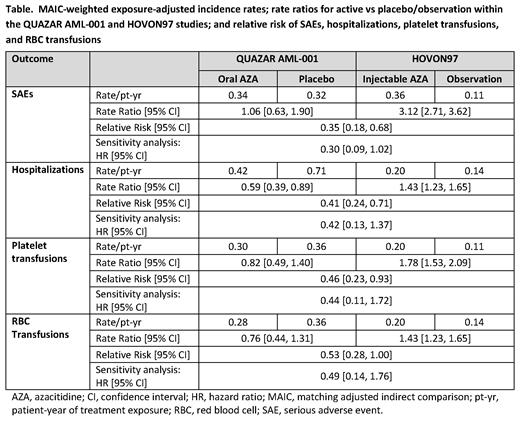
RESULTS: IPD for 398/469 pts (85%) in the QUAZAR AML-001 safety population (Oral-AZA n=203, PBO n=195) met the eligibility criteria of the HOVON97 population and were adjusted to the comparator. For all endpoints, rate ratios for Oral-AZA vs PBO were less than those for SC AZA vs observation (Table). MAIC-weighted safety comparisons showed the risk of SAEs was reduced by 65% for patients treated with Oral-AZA vs SC AZA (RR 0.35 [95% CI: 0.18-0.68]). Similarly, the risks of hospitalization requirements, platelet transfusion, and RBC transfusion were reduced with Oral-AZA vs SC AZA by 59% (RR 0.41 [95% CI: 0.24-0.71), 54% (RR 0.46 [0.23-0.93]), and 47% (RR 0.53 [0.28-1.00]), respectively. All findings were robust to the sensitivity analyses relaxing assumptions of imputed Tx exposure in HOVON97, which showed pts treated with Oral-AZA are 70% less likely to experience SAEs than pts treated with SC AZA (hazard ratio: 0.30 [95% CI: 0.09-1.02]), and hazard reductions for hospitalization and transfusions ranged from -51% to -58% (Table).
CONCLUSIONS: These data suggest that in controlled clinical trials with pts in first remission after IC, Oral-AZA maintenance therapy not only significantly improves OS, but also provides a better safety profile and requires less HCRU than SC AZA maintenance therapy. Interpretation of results should be limited to the differences in the rate to first SAE while on Tx, as stronger generalizations are limited by competing risks and informative censoring.
Chen: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Huls: Universaity Medical Center Groningen: Current Employment. Cameron: Bristol Myers Squibb: Consultancy. Suero: Bristol Myers Squibb: Consultancy. Vasconcelos: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gaugler: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Beach: Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal